PROJECTS
1. Aspen (Populus tremula) chemistry
Trees in the family Salicaceae (poplars and willows) use phenolic glycosides (salicinoids) as defensive compounds against a variety of insect and mammanlian herbivores, investing as much as 25% of their dry weight in these chemicals. We surveyed 102 replicated genotypes of European aspen (Populus tremula) from the Swedish Aspen collection (SwAsp) for foliar phenolic glycosides using UHPLC-ESI-TOF/MS and identified nine novel compounds, bringing the total to 19 for this species (Fig. 1, below). Phenolic glycoside structure followed a modular architecture of a salicin skeleton with added side groups, alone or in combination. Two main moieties, 2’-cinnamoyl and 2’-acetyl, grouped the SwAsp population into four distinct chemotypes, and the relative allocation of phenolic glycosides was remarkably constant between different environments, implying a highly channeled biosynthesis of these compounds. Slightly more than half of the SwAsp genotypes belonged to the cinnamoyl chemotype. A fraction synthesized the acetyl moiety alone (~7%) or in combination with cinnamoyl (~2%), and close to forty percent lacked either of the two characteristic moieties, and thus resemble P. tremuloides in their phenolic glycoside profile (Fig. 1, below).
Keefover-Ring, K., M. Ahnlund, I. Nacif Abreu, S. Jansson, T. Moritz, and B.R. Albrectsen. 2014. Salicinoid chemotypes show no evidence of geographical structure in Populus tremula. PLoS ONE 9(10): e107189 (Published online 9 Oct 2014) PDF
We plan to use these data as an additonal trait in a genome-wide association study with the recently sequenced SwAsp collection.
Fig. 1. Structural relationship of 19 salicinoids found in the foliage of Populus tremula from the SwAsp collection grouped similar to the loading plot (Fig. 2b). 1 = new compounds for P. tremula, 2 = compounds with two isomers present but the conformation of the cinnamoyl group double bond is ambiguous. 3 = 2’-(E)- and 2’-(Z)-cinnamoylsalicortin. PCA results for chemotypes and compound relationship of 19 salicinoids found in the foliage of Populus tremula from the SwAsp collection. a. Score scatter plot of the first two principle components for 319 individuals from 102 genotypes. Solid symbols = greenhouse grown trees, open symbols = field grown trees; circles = CN (2’-cinnamoyl), diamonds = AC (2’-acetyl), squares = CN-AC (2’-cinnamoyl/2’-acetyl), and triangles = TL (tremuloides-like) chemotypes. Percentages of clones of the different salicinoid chemotypes in parentheses. b. Loading scatter plot of the first two principle components for 19 salicinoids.
We also conclusively identified one of the new and very abundant P. tremula phenolic glycosides (2"-(Z)-cinnamoylsalicortin, see Fig. 1, below) with MS/MS and NMR:
Keefover-Ring, K., M. Carlsson, and B.R. Albrectsen. 2014. 2'-(Z)-Cinnamoylsalicortin: A novel salicinoid from Populus tremula. Phytochemistry Letters 7:212-216 (Published online 13 Dec 2013) PDF
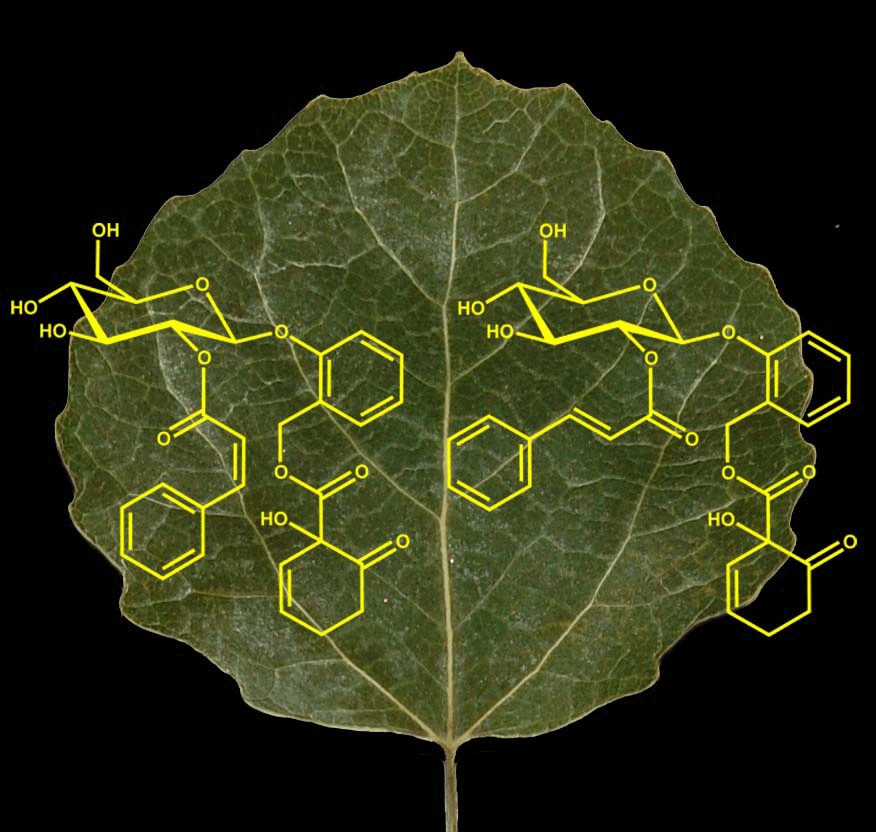
Fig. 1. 2"-(Z)-cinnamoylsalicortin (left), a novel phenolic glycoside from the foliage of Populus tremula. An isomer of the known bioactive compound 2"-(E)-cinnamoylsalicortin (right)
2. Pine Chemical Ecology
Cone insects and pine chemistry
For my Master's degree at the University of Colorado at Boulder I studied terpenoid chemistry and herbivores [see the cone moth (Dioryctria spp.) on the banner photo of my Publications page] in the seed cones of ponderosa pine (Pinus ponderosa). This specialized group of insects lays eggs in or on green second-year cones and their larvae mine the cones' interior, destroying seeds and affecting tree reproduction (see Fig. 2, below). We measured the terpenoid chemistry, cone insect distribution, and the relationship between these two parameters in the seed cones of ponderosa pine. Analyses of mono-, sesqui-, and diterpenes from four separate sites revealed high amounts of terpenoid diversity and variation. The majority of this variation occurred among trees within sites, but differences were also seen among sites and among cone clusters from individual trees. Cone insect distributions differed substantially in both time and space, with significant differences seen between two points in time and between five sites. Negative correlations existed between levels of cone insect herbivory and both a monoterpene and a diterpene factor at one site, indicating that these herbivores may be one reason that ponderosa pine maintains the high levels of chemical variation observed.
Keefover-Ring K. and Linhart Y.B. 2010. Variable chemistry and herbivory in ponderosa pine cones. International Journal of Plant Sciences 171(3): 293-302 PDF

Fig. 2. Healthy (left) and cone insect-infested (right) cones and examples of the two main classes of terpenoids (mono- and diterpenes) from ponderosa pine
Degradation of pine terpenes by bacteria associated with bark beetles
In a collaboration with the Prof. Ken Raffa lab at University of Wisconsin-Madison, we showed that bacteria associated with mountain pine beetles can metabolize monoterpenes and diterpene acids. The abilities of different symbionts to reduce concentrations of different terpenes appeared complementary (Fig. 3, below). Serratia reduced concentrations of all monoterpenes applied to media by 55–75 %, except for α-pinene. Beetle-associated Rahnella reduced (−)- and (+)-α-pinene by 40 % and 45 %, respectively. Serratia and Brevundimonas reduced diterpene abietic acid levels by 100 % at low concentrations . However, high concentrations exhausted this ability, suggesting that opposing rates of bacterial metabolism and plant induction of terpenes are critical.
Boone, C.K., K. Keefover-Ring, A.C. Mapes, A.S. Adams, J. Bohlmann, and K.F. Raffa. 2013. Bacteria associated with a tree-killing insect reduce concentrations of plant defense compounds. Journal of Chemical Ecology 39:1003–1006 PDF

Fig. 3. Degradation of various monoterpenes and the diterpene abietic acid by bark beetle-associated bacteria
3.Thyme (Thymus sp.) Chemical Ecology
The genus Thymus is an Old World endemic with the center of diversity found in the Western Mediterranean. In southern France thyme occurs in an ecosystem called garrigue, a mixture of oak and pine woodlands with open areas where thyme and many other labiates are found. For the past 40 years the most extensive research on Thymus vulgaris has been conducted in an area north of Montpellier, France and at the Centre d’Ecologie Fonctionnelle et Evolutive (CEFE) (Thompson et al. 1998). The CEFE (http://www.cefe.cnrs.fr/) is just one institute of several on the campus of the Centre National de la Recherche Scientifique (CNRS) at Montpellier.
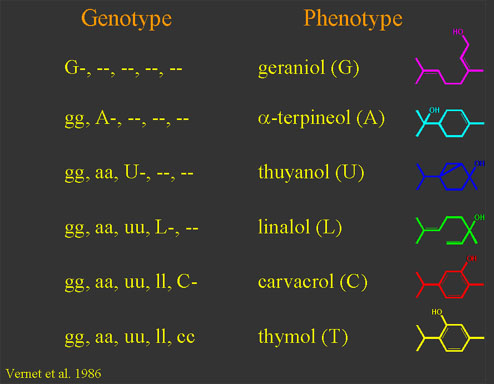 Monoterpene synthesis in thyme involves a well-documented polymorphism where individual plants produce one of six monoterpenes: geraniol (G), a-terpineol (A), thuyanol (U), linalol (L), carvacrol (C), or thymol (T) as the dominant component of their total monoterpene profile (Vernet et al. 1986). The genetic control involves an epistatic series of at least five loci. At each locus, there is a pair of alleles, one dominant to the other. The epistatic effects are linear, so that: G > A > U > L > C > T. Therefore, a plant that has at least one dominant allele at the G locus produces a G chemotype regardless of its genetic composition at the other loci. A plant that is homozygous recessive at the G locus (i.e. gg) but with at least one dominant A allele will have chemotype A, again regardless of the genetic composition of the remaining loci in the series. The series continues in this manner. A plant which is homozygous recessive at all five loci has chemotype T. We also recently discovered a T. vulgaris chemotype new to this well-studied area in southern France which contains 1,8-cineole (eucalyptol) as its dominant monoterpene.
Monoterpene synthesis in thyme involves a well-documented polymorphism where individual plants produce one of six monoterpenes: geraniol (G), a-terpineol (A), thuyanol (U), linalol (L), carvacrol (C), or thymol (T) as the dominant component of their total monoterpene profile (Vernet et al. 1986). The genetic control involves an epistatic series of at least five loci. At each locus, there is a pair of alleles, one dominant to the other. The epistatic effects are linear, so that: G > A > U > L > C > T. Therefore, a plant that has at least one dominant allele at the G locus produces a G chemotype regardless of its genetic composition at the other loci. A plant that is homozygous recessive at the G locus (i.e. gg) but with at least one dominant A allele will have chemotype A, again regardless of the genetic composition of the remaining loci in the series. The series continues in this manner. A plant which is homozygous recessive at all five loci has chemotype T. We also recently discovered a T. vulgaris chemotype new to this well-studied area in southern France which contains 1,8-cineole (eucalyptol) as its dominant monoterpene.
Keefover-Ring K., J.D. Thompson, and Y.B. Linhart 2009. Beyond six scents: defining a seventh Thymus vulgaris chemotype new to southern France by ethanol extraction. Flavour and Fragrance Journal 24: 117-122 (Published online 12 Feb 2009) PDF
These various chemotypes have been shown to have differential effects on many other community memebers (Linhart and Thompson 1995, 1999). For instance, in one experiment where we grew four different thyme monoterpene chemotypes in a 4 x 2 x 2 factorial of chemotype, caging (sham half-cages vs. full cages), and competition (control vs. the grass Bromus madritensis L.), we found that thyme-feeding aphids (Aphis serpylli Koch) passed through full cage walls to increase more than fourfold on all but one chemotype (Fig. 4, below).
Linhart Y.B., K. Keefover-Ring, K.A. Mooney, B. Breland , and J.D. Thompson. 2005. A chemical polymorphism in a multi-trophic setting: thyme monoterpene composition and food web structure. American Naturalist 166(4): 517-529 (Published online 5 Aug 2005) PDF
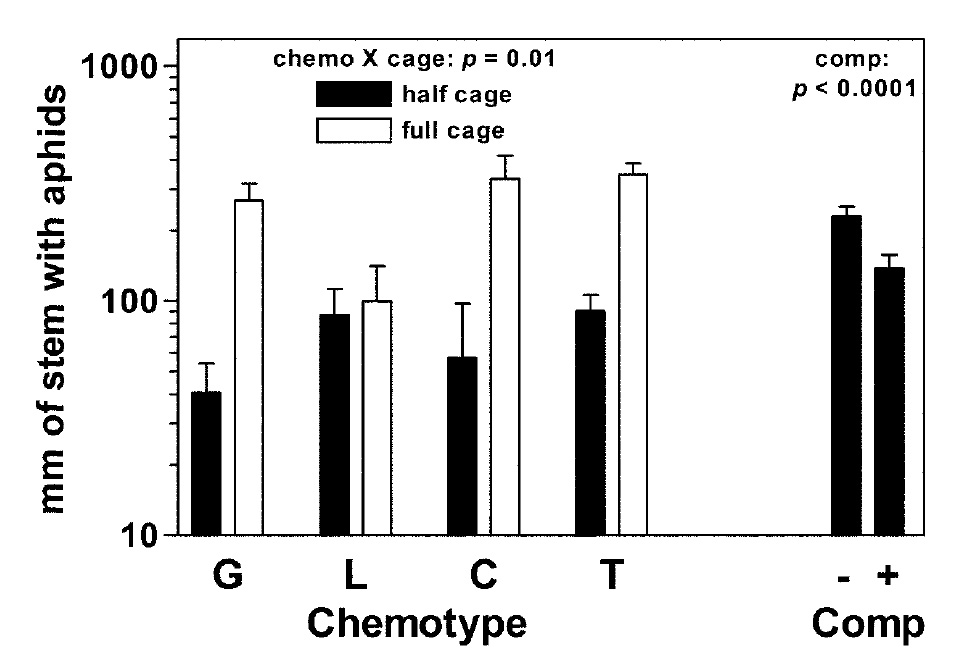
Fig. 4. Aphid (Aphis serpylli) abundance on thyme in chemotype, predator exclusion (cage), and competition (comp) treatments. Aphid abundances within cage and chemotype treatments are shown together because these two factors interacted significantly. Competition did not interact with other treatments
Thyme terpenes can also be allelopathic (inhibit the growth of other plants). We have shown that the foliage of some chemotypes can inhibit the germination of associated species, although, the effect differed depending upon the source of the soil used to grow the associated species (from under or away from thyme plants; Fig. 5, below).
Linhart, Y.B., P. Gauthier, K. Keefover-Ring, and J.D. Thompson. 2014. Variable phytotoxic effects of Thymus vulgaris (Lamiaceae) terpenes upon associated species. International Journal of Plant Sciences (in press)
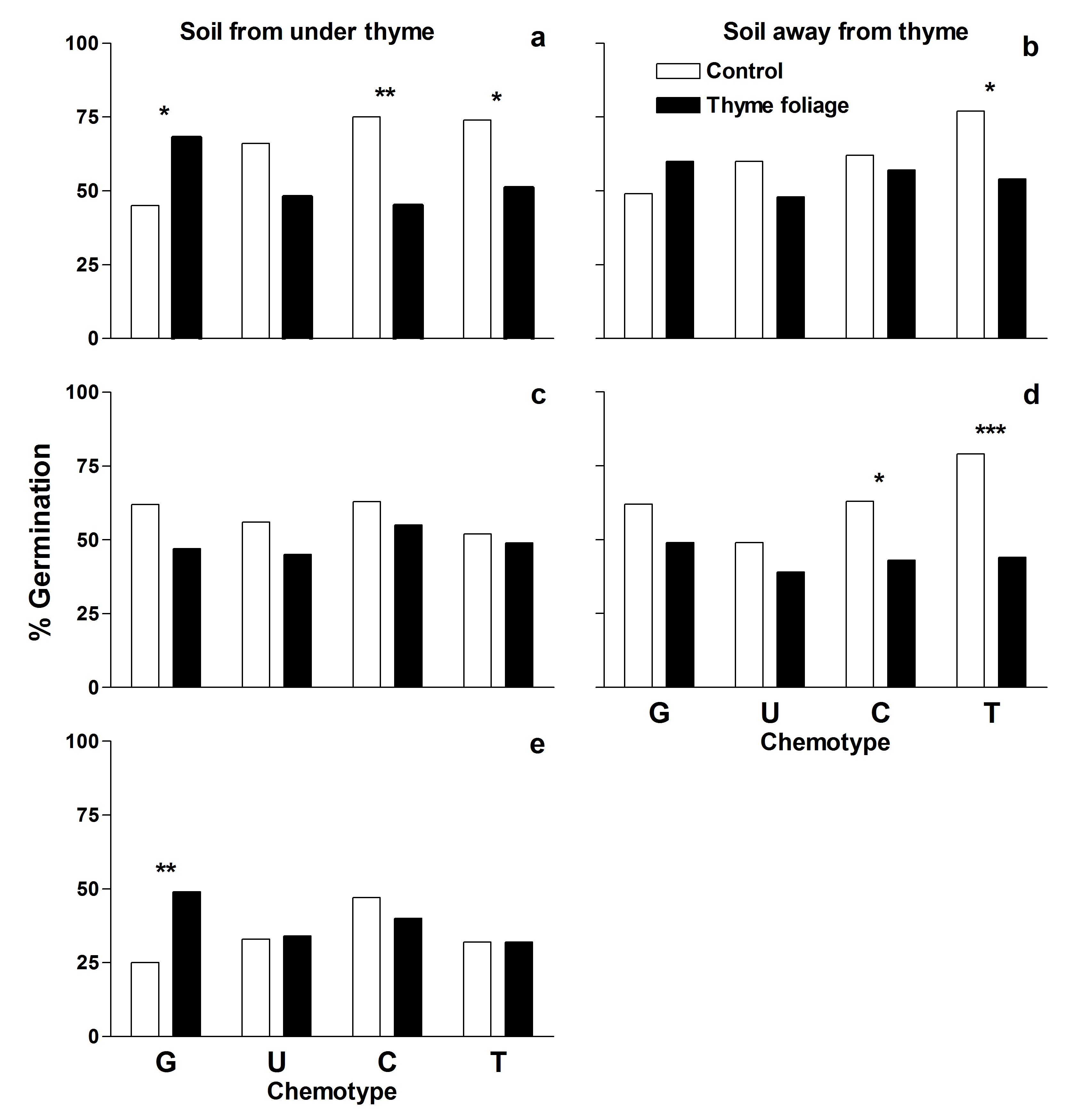
Fig. 5. Percent germination for Daucus carota (a and b) and Nigella damascena (c and d) grown in soil collected either away or from under thyme plants of different chemotypes, and Bromus madritensis (e) only in soil from under thyme plants of different chemotypes, without (control, white bars) or with thyme foliage (black bars). G = geraniol. U = cis-sabinene hydrate, C = carvacrol, and T = thymol. * = 0.05 < p < 0.01, ** = 0.01 < p < 0.001, *** = p < 0.001
4. Mimulus guttatus (yellow monkeyflower) Secondary Chemistry
Yellow monkeyflower [Mimulus guttatus DC., (Phyrmaceae)] has long been a model plant species for studies in genetics, evolution, and ecology, including plant-animal interactions. Nonetheless, exceedingly little is known about its secondary chemistry. We have discovered that the foliage of yellow monkeyflower contains a diverse suite of phenylpropanoid glycosides (PPGs); a class of compounds with many known biological activities.
We intially looked at the patterns of these compounds in yellow monkeyflower from several different natural populations and found differences between populations and between different life histories (annual or perennial, Fig. 6, below):
Holeski, L.M., K. Keefover-Ring, M.D. Bowers, Z.T. HarnEnz, and R.L. Lindroth. 2013. Patterns of phytochemical variation in Mimulus guttatus (yellow monkeyflower). Journal of Chemical Ecology 39: 525-536 (Published online 7 Mar 2013) PDF
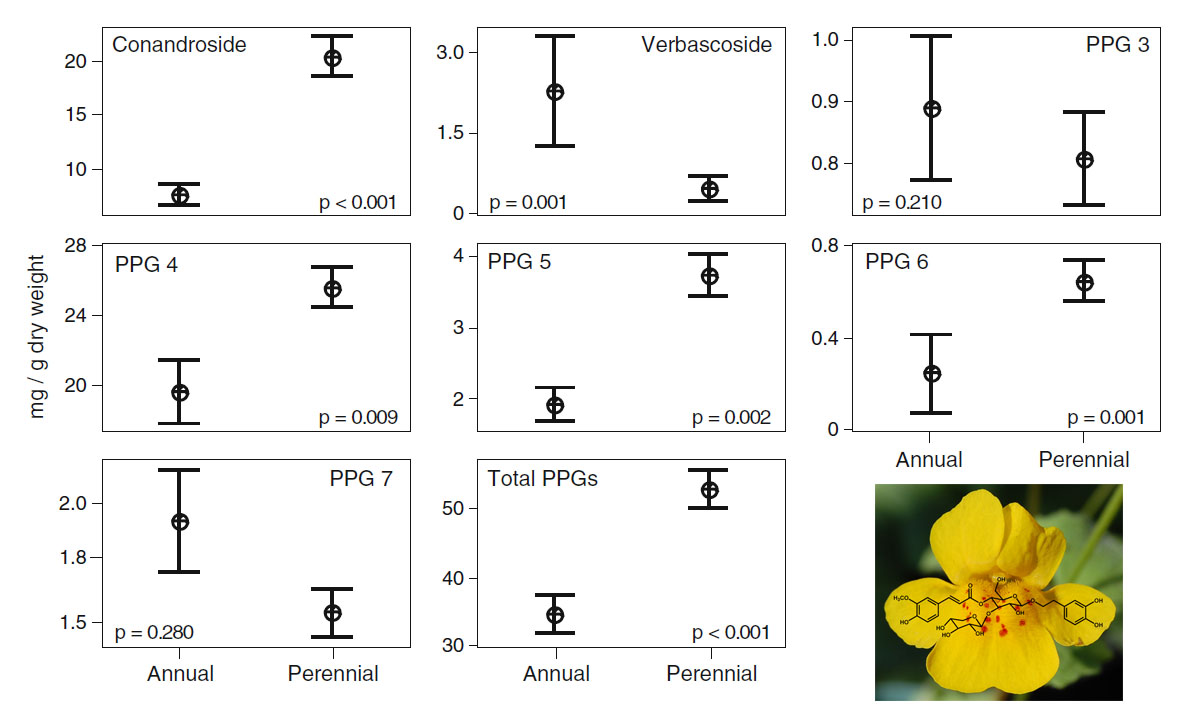
Fig. 6. Levels of PPGs in annual and perennial yellow monkeyflower
We used 1H and 13C NMR and UV and MS chromatography techniques to positively identify five PPGs from the leaves of yellow monkeyflower (Fig. 7, below). Four of these compounds occur in other species and one is previously undescribed [mimuloside (5)]. We also presented UV and high-resolution tandem MS data that putatively identify 11 additional foliar compounds as PPGs. This initial discovery and elucidation of yellow monkeyflower’s secondary chemistry will be important for continued study of the genetics and ecology of this model species.
Keefover-Ring K., Holeski, L.M., Bowers, M.D., Clauus, A.D., and Lindroth R.L. 2014. Phenylpropanoid chemistry of Mimulus guttatus (yellow monkeyflower). Phtyochemisty Letters (Published online 4 Sep 2014) PDF - supplmentary material

Fig. 7. Five identified PPGs from the foliage of yellow monkyflower. Mimuloside (5) is a new PPG
5. Insect Use of Host Chemistry
Tortoise beetles using host terpenes
The larvae of many species of tortoise beetle protect thmeselves with a structure called a fecal shield (lower right photo in Fig. 8, below). Larvae, such as these of Physonota unipunctata, feed on plants high in secondary compounds and incorporate these chemicals into their fecal shields for defense. However, often these compounds are volatile and may be used by parasatoids to find their host. See how these larvae concentrate the more toxic monoterpenes from their host (Monarda fistulosa) into their shileds, but also cause large amounts of volatile emissions when feeding.
Keefover-Ring K. 2013. Making scents of defense: do fecal shields and herbivore-caused volatiles match host plant chemical profiles? Chemoecology 23:1-11 PDF

Fig. 8. Life history of Physonota unipunctata (clockwise from top left): Adult, ootheca, first instars just after hatching, and last instars with fecal shields